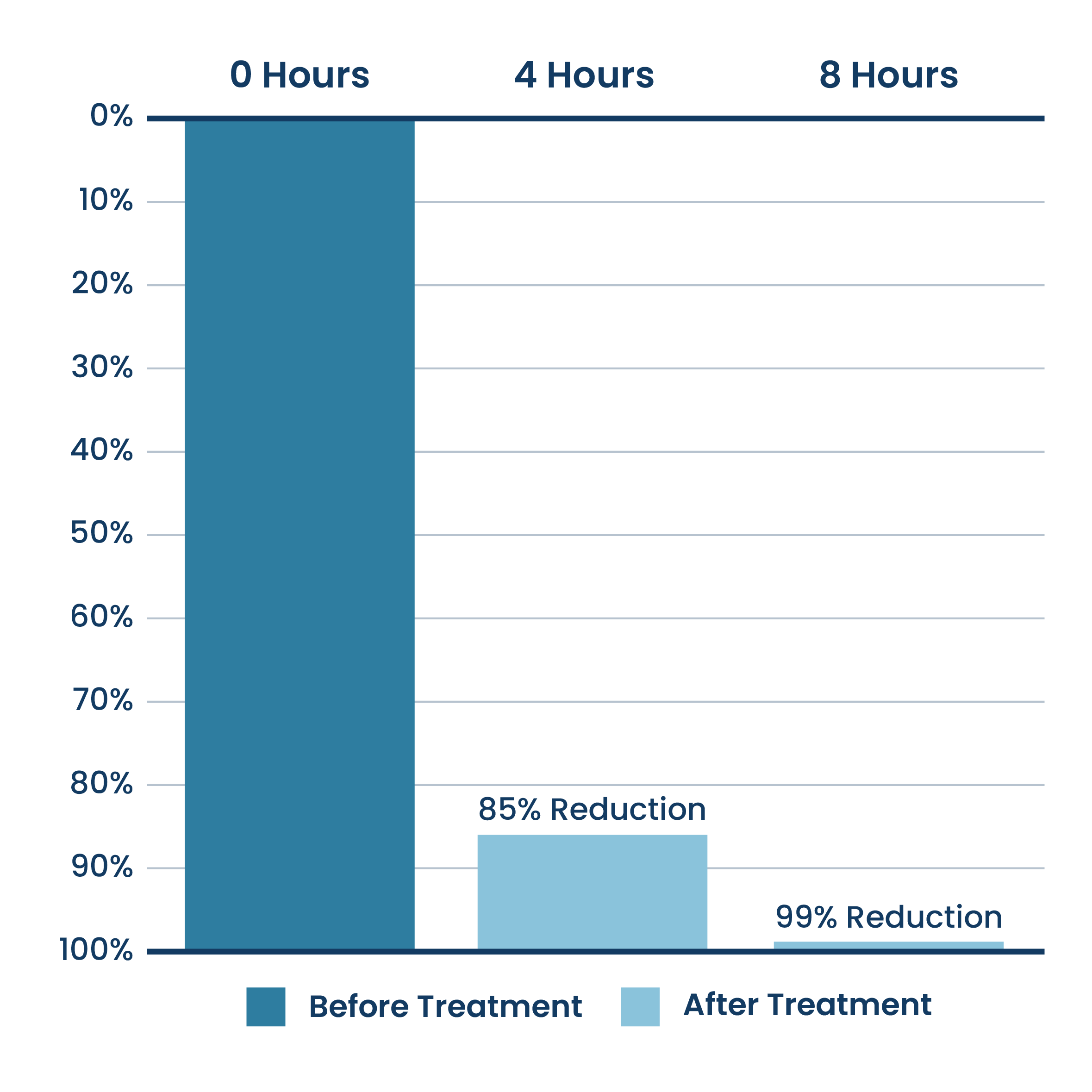
ActivePure Hydroxyl Surface Efficacy Data

View All











ActivePure® SARS-CoV-2 Testing
View All
ActivePure® SARS-CoV-2 Testing
(The Virus That Causes COVID-19)
Testing of the SARS-CoV-2 virus was completed in August 2020 (surface) and in December 2020 (airborne). The results of the laboratory testing revealed extraordinary reduction levels of the virus that causes COVID-19.
Average Percent SARS-CoV-2 Airborne Reduction
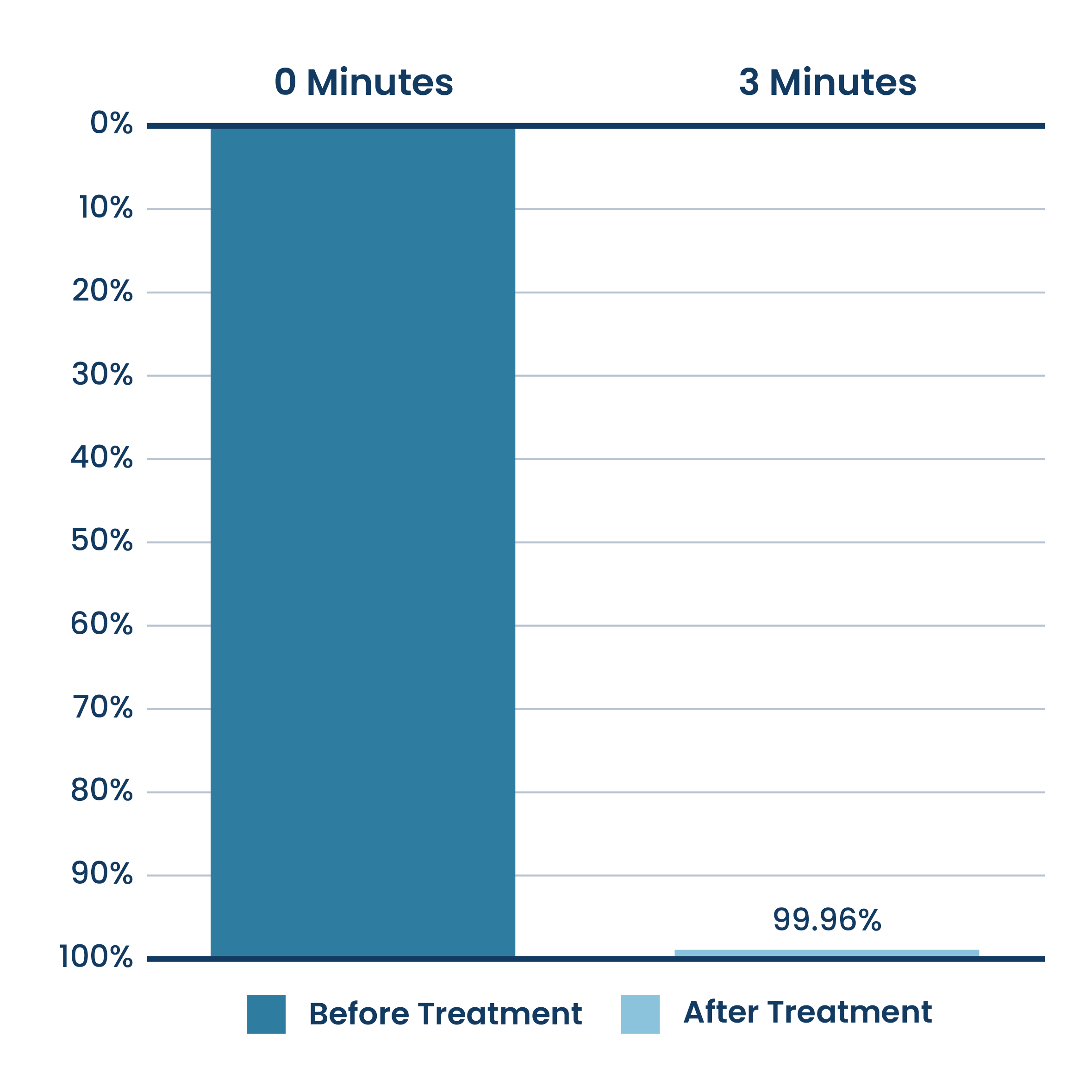
New Pure & Clean Unit Testing
Testing done at a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates.
Average Percent SARS-CoV-2 Reduction On Surfaces

Aerus Hydroxyl Blaster with ActivePure® Technology Unit Testing.
Testing was done at MRIGlobal, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates. The reduction percentages were measured incrementally over natural degradation of SARS-CoV-2. Outside of control group – over 99.9% reduction of the SARS-CoV-2 virus.
ActivePure® Pathogen & Contaminants Testing
Laboratory testing results of the pathogens and contaminants listed below revealed extraordinary levels of reduction using ActivePure Technology.
Airborne Reduction Of Influenza (H5N8 / Avian-Bird Flu)

New ActivePure® Medical Unit Testing.
Results are based on laboratory testing conducted to demonstrate the use of ActivePure® Technology in substantially reducing airborne contaminants. Field results may vary based on environmental conditions. These results have not been evaluated by the FDA.
Airborne Reduction Of RNA Virus (MS2 Bacteriophage)
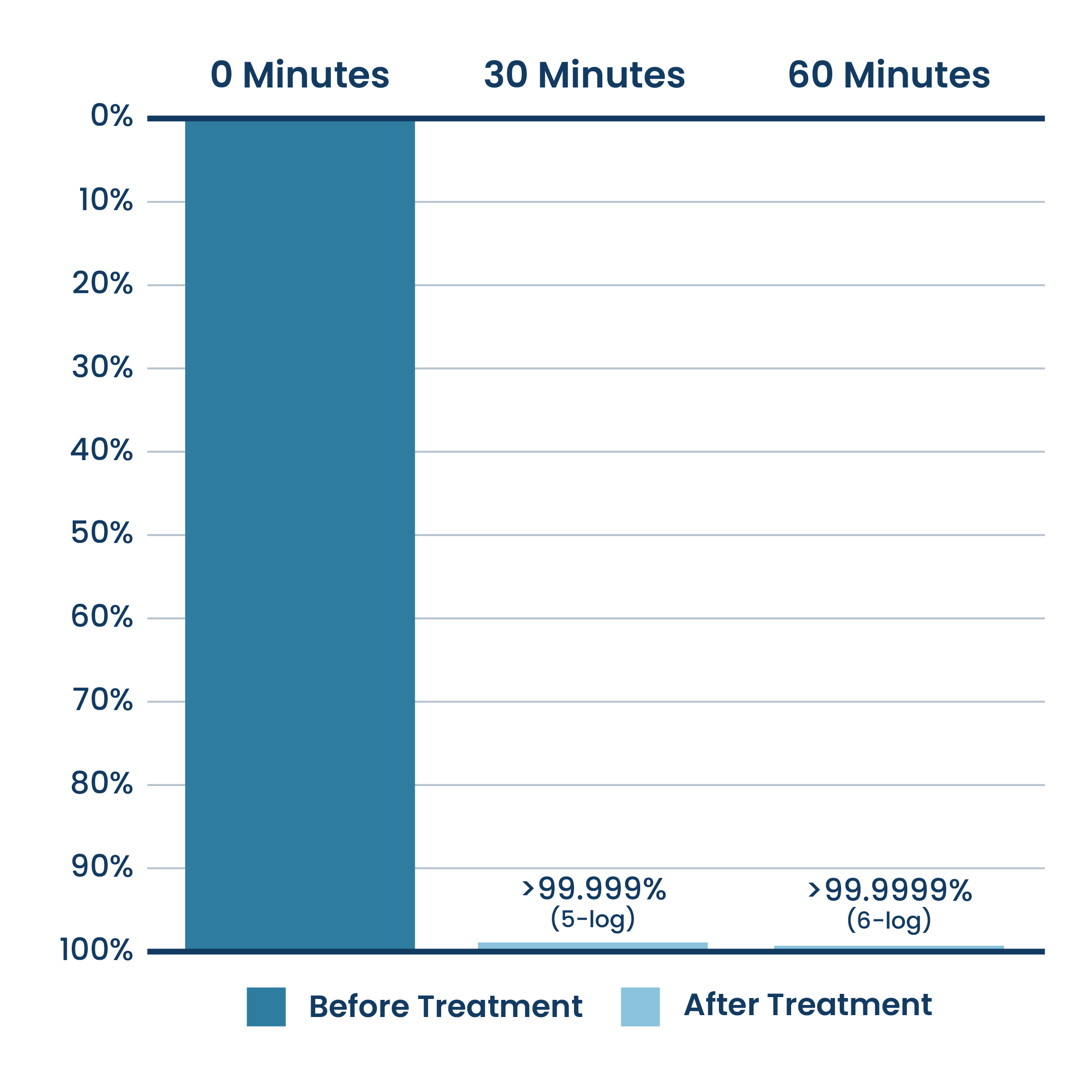
New ActivePure® Medical Unit Testing.
Testing done at Aerosol Research and Engineering Laboratories, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates. Net Log Reduction of MS2 Bacteriophage Virus Bioaerosol.
Airborne Reduction Of DNA Virus (Phi-X174 Bacteriophage)
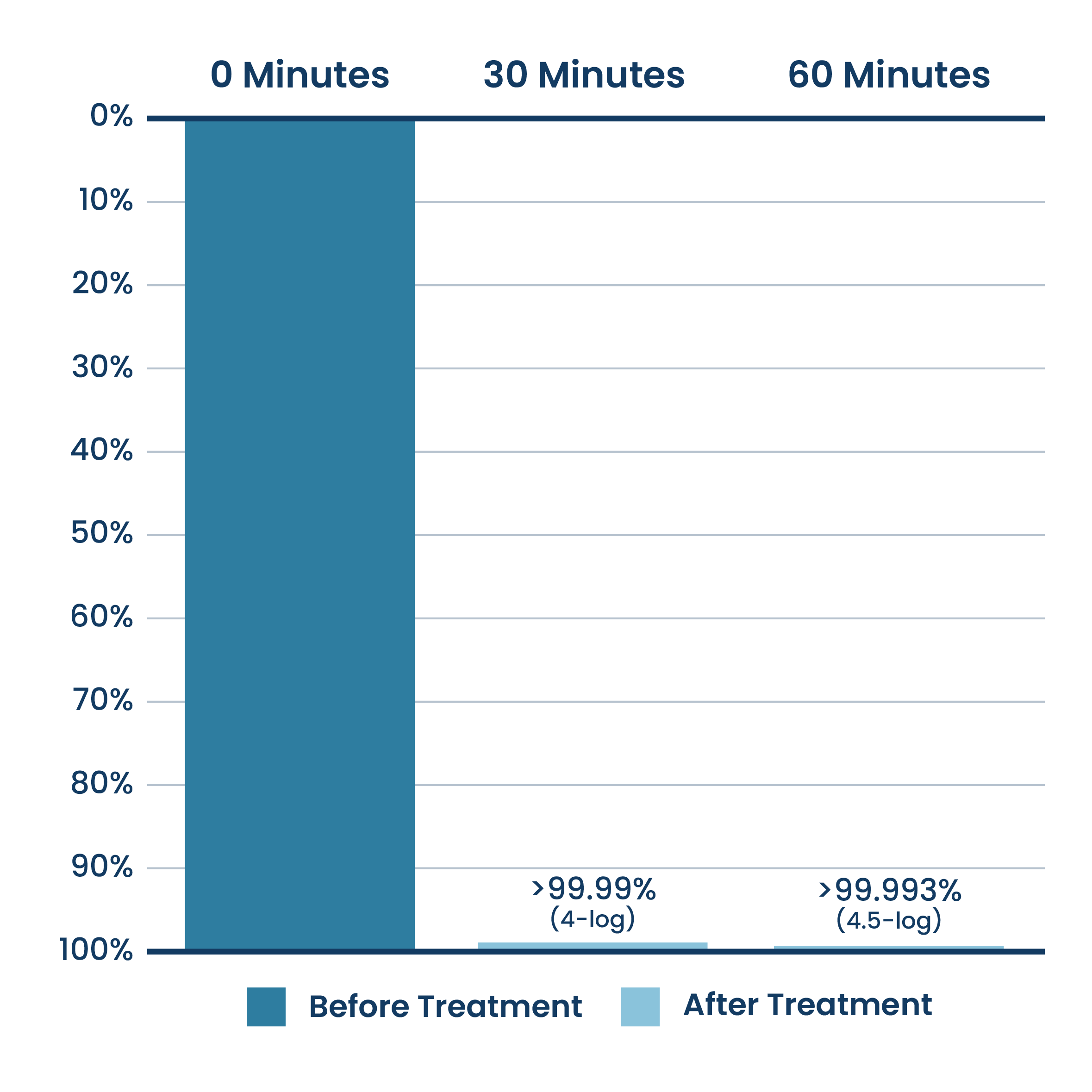
New ActivePure® Medical Unit Testing.
Testing done at Aerosol Research and Engineering Laboratories, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates. Net Log Reduction Phi-X174 of Bacteriophage Virus Bioaerosol.
Airborne Reduction Of H1N1 Virus (Swine Flu Microbes) 
New ActivePure® Medical Unit Testing.
Results are based on laboratory testing conducted to demonstrate the use of ActivePure® Technology in substantially reducing airborne contaminants. Field results may vary based on environmental conditions. These results have not been evaluated by the FDA.
Volatile Organic Compounds (TVOC Reduction)
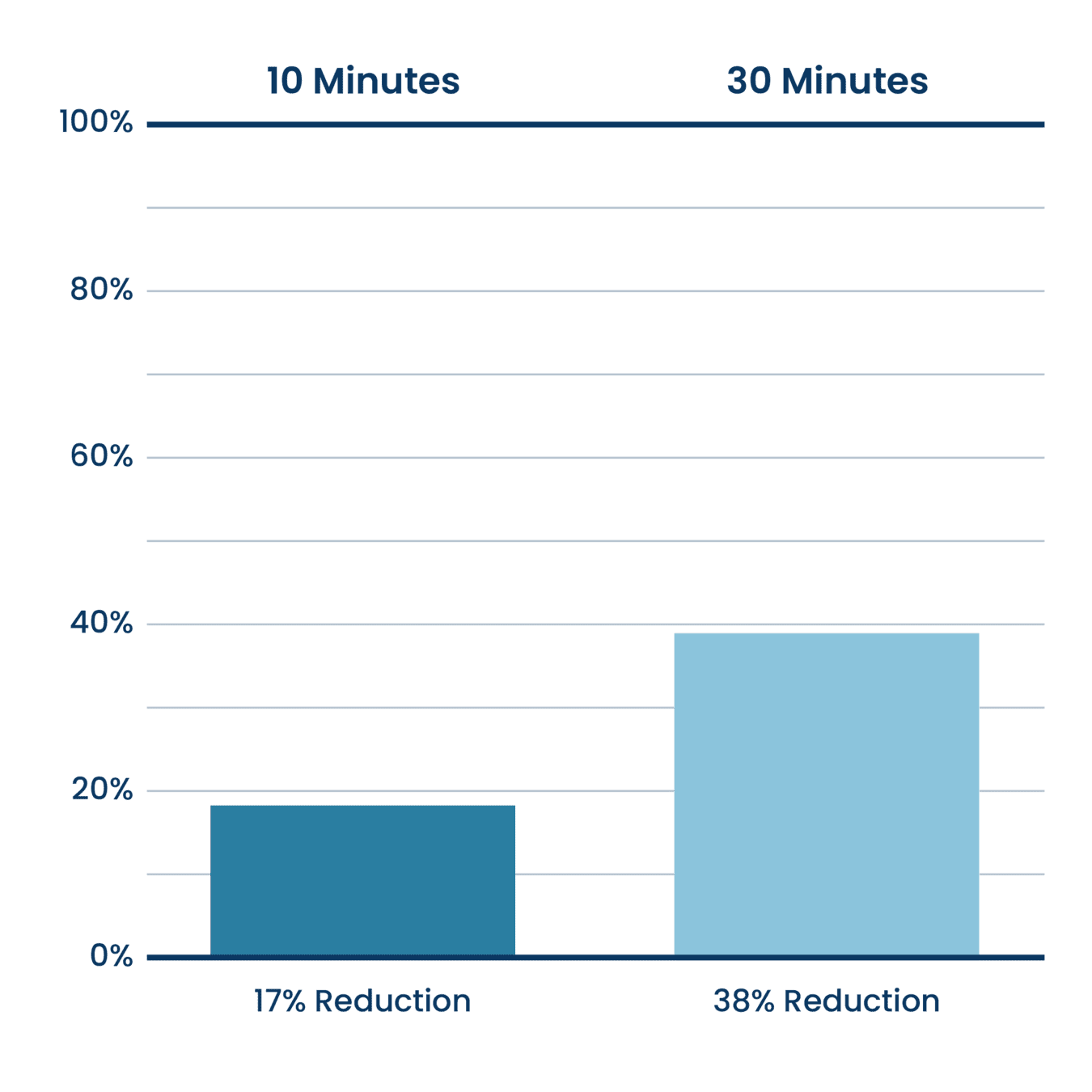
Aerus Beyond Guardian Unit Testing.
Testing done at Danish Technological Institute, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates. Testing was completed with the air purifier installed within a seal 20 cubic meter room. Experiment was designed to measure the reduction rate during use.
Cigarette Smoke Reduction (Particulate Matter <0.8 Microns)
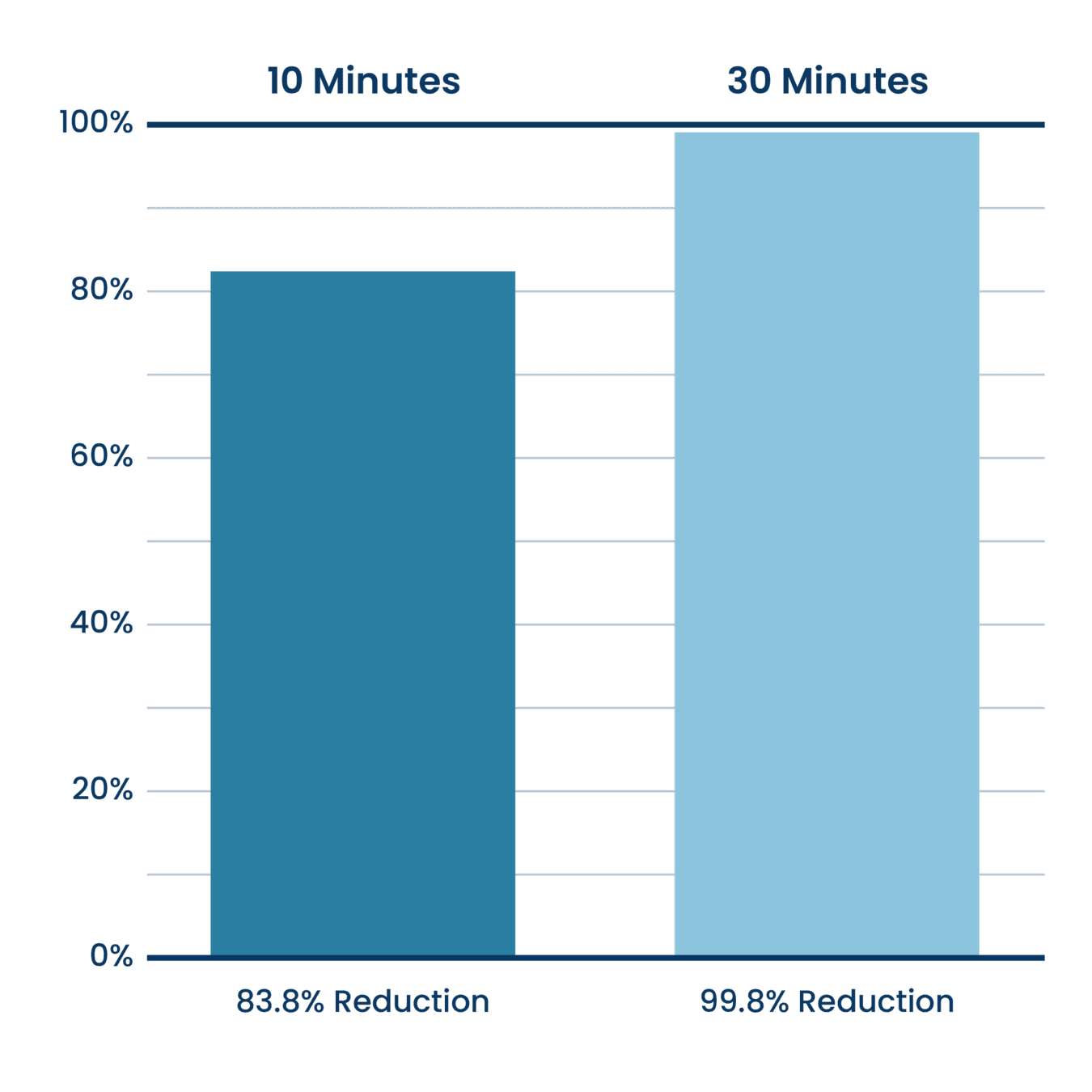
Aerus Beyond Guardian Unit Testing.
Testing done at Danish Technological Institute, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates. Testing was completed with the air purifier installed within a seal 20 cubic meter room. Experiment was designed to measure the reduction rate during use.
Cigarette Smoke Reduction (Particulate Matter 2.5 Microns)
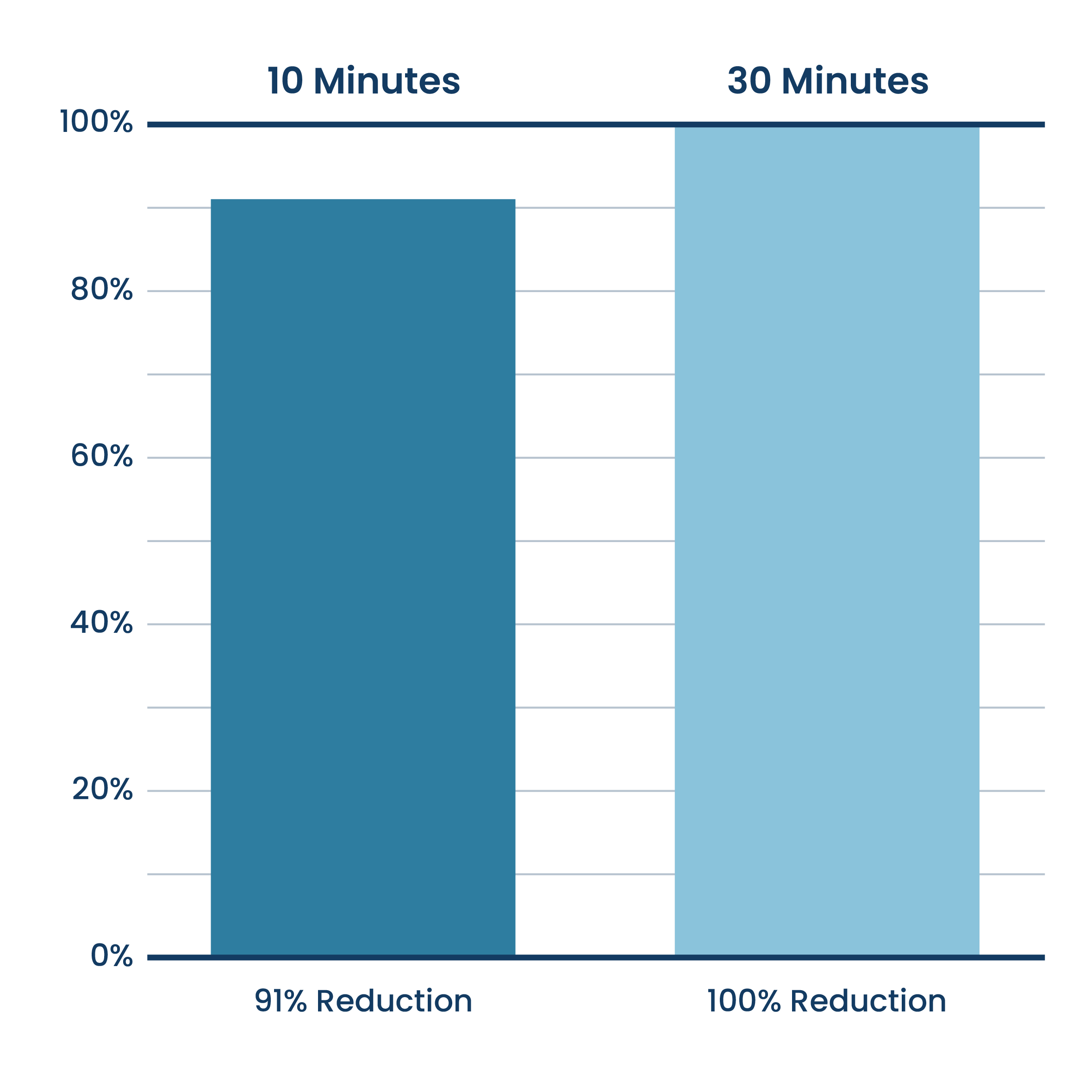
Aerus Beyond Guardian Unit Testing.
Testing done at Danish Technological Institute, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates.Testing was completed with the air purifier installed within a seal 20 cubic meter room. Experiment was designed to measure the reduction rate during use.
Surface Reduction Of Bacteria (Within Hotel Facilities)
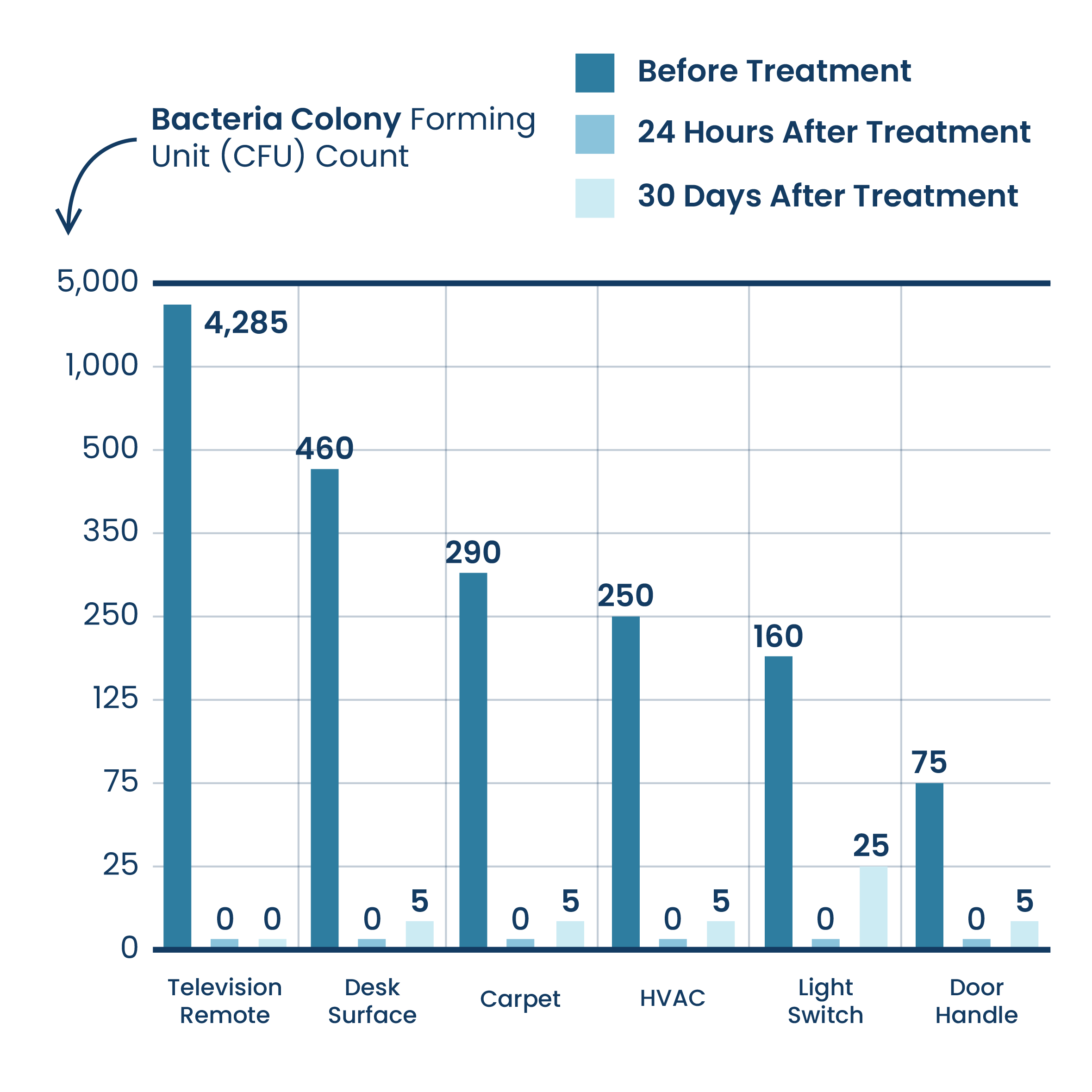
Results are based on testing conducted using ActivePure® Technology within a Hotel Facility. The results are combined from testing conducted within 3 rooms.
Surface Reduction Of Fungi (Within Hotel Facilities)
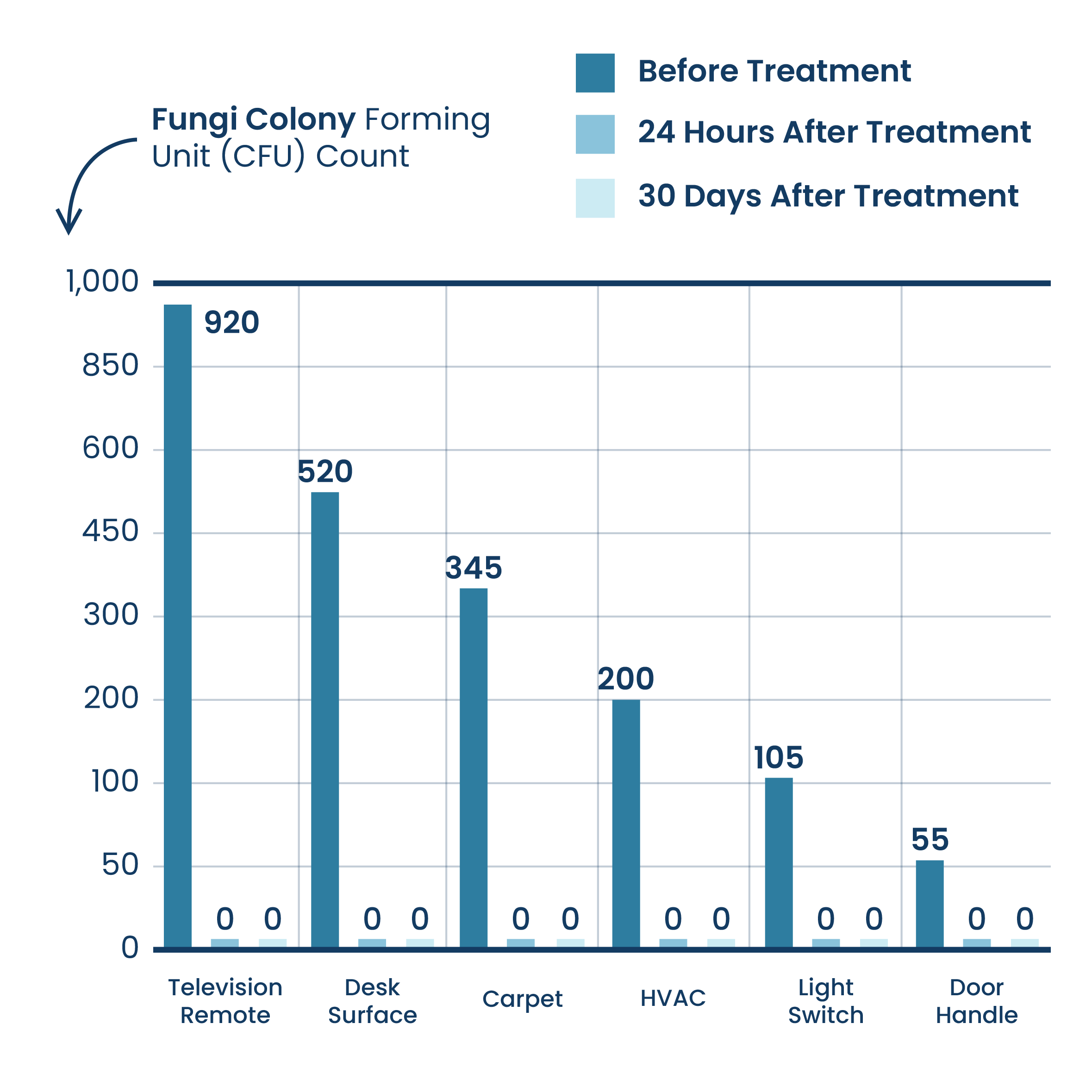
Results are based on testing conducted using ActivePure® Technology within a Hotel Facility. The results are combined from testing conducted within 3 rooms.
Reduction Of Airborne Fungal Mold (Aspergillus Niger)

New ActivePure® Medical Unit Testing.
Testing done at Aerosol Research and Engineering Laboratories, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates. Net Log Reduction of Aspergillus Niger (fungal spores).
Reduction Of Airborne Gram-Positive Bacteria Bioareosol (Staphylococcus Epidermidis)
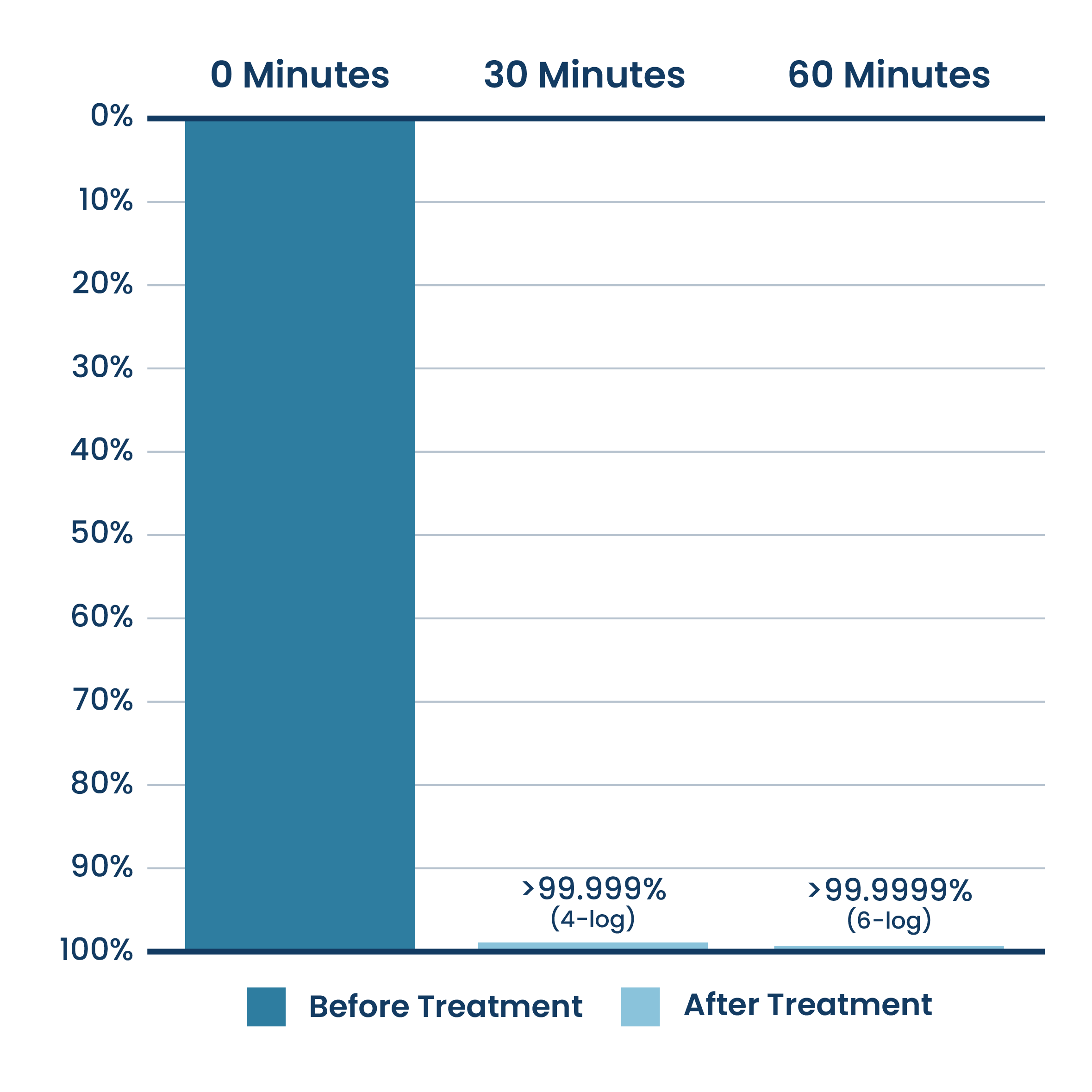
New ActivePure® Medical Unit Testing.
Testing done at Aerosol Research and Engineering Laboratories, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates. Net Log Reduction of Staphylococcus Epidermidis Bioaerosol.
Reduction Of Airborne Gram-Negative Bacteria Bioareosol (Erwina Herbicola)

New ActivePure® Medical Unit Testing.
Testing done at Aerosol Research and Engineering Laboratories, a 3rd party unaffiliated laboratory with no connection to ActivePure or its affiliates. Net Log Erwinia Reduction of Bacillus Globigii (bacterial spores).
The Pathogens ActivePure® Can Combat Against:
- SARS-CoV-2
- Staphylococcus Aureus
- Aspergillus Niger Endospores (Toxic Black Mold Surrogate)
- H1N1 Influenza (Swine Flu)
- H5N8 Influenza (Bird Flu)
- MRSA (Methicillin-Resistant)
- Bacillus globigii (C. Difficile And Anthrax Surrogate)
- MS2 Bacteriphage – RNA Virus
- Staphylococcus epidermidis
- Phi-X174 – DNA Virus
- Erwinia Herbicola
- Listeria Monocytogenes
- Escherichia Coli (E. Coli)
- Candida Auris (Fungus)
- Sclerotinia Sclerotiorum
- Legionella Pnuemophila
- Aspergillus Versicolor
- Clostridium Difficile
- Salmonella Enterica
Superior Indoor Air Quality Starts Here
Improving your indoor air quality has never been easier. Our portable air purifier systems are fully customizable and scalable in design and can cover any indoor space, regardless of size. Explore our portable air purifier solutions.
Available ActivePure® Products
Please contact me for more information or to purchase:
Dave Donovan: 403.605.4177
info@bluesteamtech.ca